

We’re proud to announce that UT Southwestern’s Robert D. Rogers Advanced Comprehensive Stroke Center has again received The Joint Commission’s Gold Seal of Approval recertification for the fifth consecutive time. UTSW has held the Commission’s highest level of certification for stroke care since 2014.
“Achieving and maintaining this level of stroke care for a large referral center like UT Southwestern is a demonstration of teamwork on many levels,” said Stroke Center Co-Director Babu Welch, M.D., Professor of Neurological Surgery and Radiology.
Co-Director Parth Upadhyaya, D.O., Assistant Professor of Neurology, agrees: “This certification highlights a culture of coordinated care in the areas of neurosurgery, neurology, radiology, neurocritical care, and rehabilitation that has existed for decades. We are proud to continue the tradition.”
UT Southwestern offers the highest level of stroke care for all types and stages of strokes. As the first Joint Commission-certified Advanced Comprehensive Stroke Center in North Texas, UTSW offers the advantages of dedicated neurointensive care unit beds that provide around-the-clock neurocritical care for patients with complex cerebrovascular disease; advanced imaging capabilities, available at all times; coordinated posthospital care; and participation in ongoing stroke research.
The certification (and recertification) represents UT Southwestern’s commitment to upholding the highest standards of health care quality and safety for our patients, workforce, and community. The recertification went into effect March 13 and is customarily valid for 24 months.
Research and Clinical Trials
As one of the nation’s top 10 centers for neurological care as ranked by U.S. News & World Report, with an additional designation of “High Performance” in stroke, UT Southwestern is dedicated to developing innovative treatments for cerebrovascular diseases. To that end, research is key.
Dr. Welch and his collaborators have recently published:
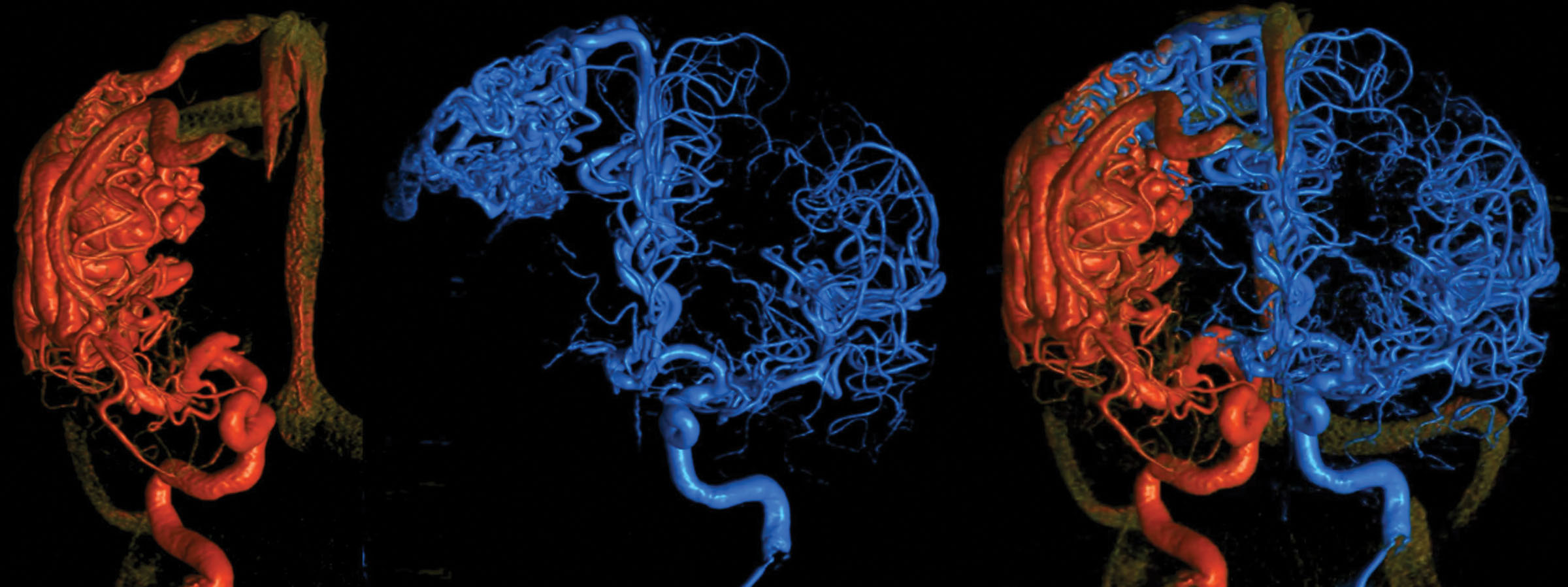
Figure 1. Six-dimensional reconstruction of a large right hemispheric arteriovenous malformation. A bihemispheric 3-dimensional rotational angiogram is fused to produce a 6-dimensional view of the entire cerebral vasculature. The super-imposition of images allows a detailed characterization of the brain arteriovenous malformation angioarchitecture and flow dynamics of different vascular territories. Images courtesy of Eytan Raz, MD, and Maksim Shapiro, MD, NYU Langone Medical Center.
- “Stereotactic Radiosurgery Versus Observation for Intracranial Low-Grade Dural Arteriovenous Fistulas,” which was a multicenter analysis of Consortium for Dural Arteriovenous Fistula Outcomes Research and International Radiosurgery Research Foundation data. The analysis found that SRS was associated with low complication risk and advised future trials on SRS as a first-line intervention for symptomatic low-grade dAVFs.
- “Most Promising Approaches to Improve Brain AVM Management: ARISE I Consensus Recommendations.” Brain AVMs (bAVMs) are rare and complex vascular lesions with no official consensus on how to manage them. ARISE (Aneurysm/bAVM/cSDH Roundtable Discussion With Industry and Stroke Experts) recognized this challenge and identified the need to develop a method for predicting the risk of bAVM rupture, initiating a multicenter collaborative effort to improve bAVM characterization, evaluation, and management. This publication shares the initial findings and ARISE consensus.
- “Five-Year Results of the SCENT Trial with Surpass Flow Diverters to Treat Large or Giant Wide-Neck Aneurysms,”which demonstrated the long-term safety and effectiveness of the Surpass flow diverter for intracranial aneurysm treatment.
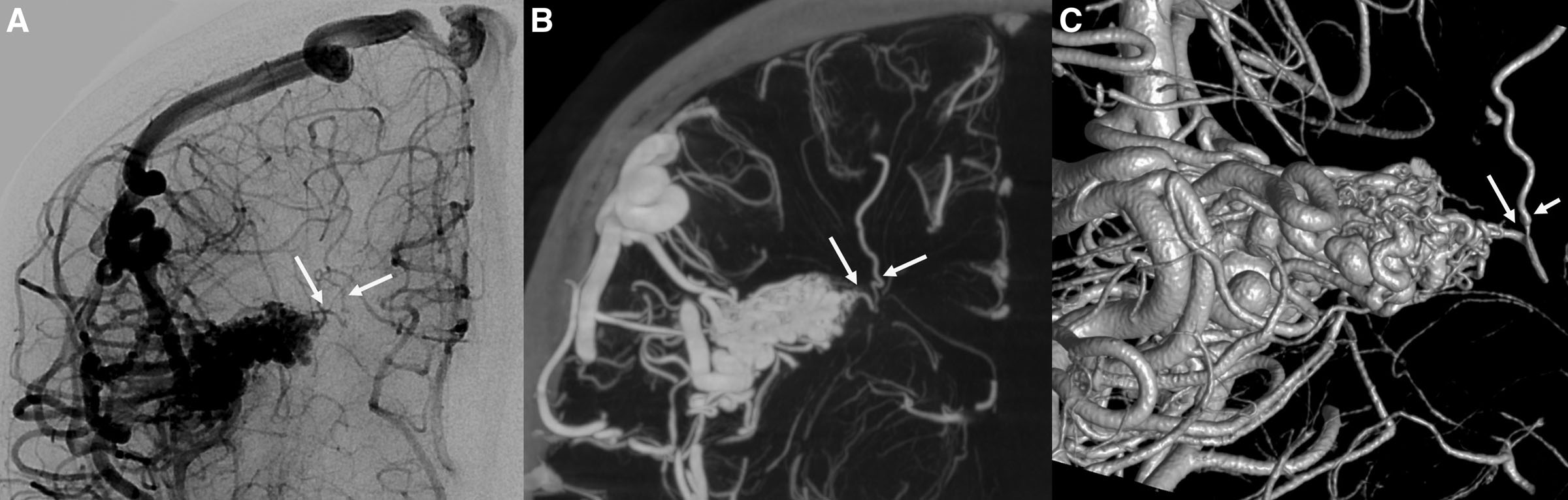
Figure 2. Digital subtraction angiography and cone beam computed angiography of a right hemispheric brain arteriovenous malformation. A, Diagnostic cerebral angiogram shows the nidus and no evidence of deep venous drainage. B, Cone beam computed angiography demonstrates the presence of a small deep vein, which can be seen more clearly in the 3-dimensional reconstruction (C, arrows). Images courtesy of Eytan Raz, MD, and Maksim Shapiro, MD, NYU Langone Medical Center.
Dr. Upadhyaya and his collaborators have recently published “Technical Considerations in the TCD Evaluation of Cerebral Circulatory Arrest in Death by Neurological Criteria.” This study analyzed the technical details and clinimetric properties of transcranial Doppler ultrasound (TCD) as an ancillary test for diagnosing brain death/death by neurologic criteria (BD/DNC) and found significant inconsistencies and lack of details in the available literature.
The Robert D. Rogers Advanced Comprehensive Stroke Center is involved in two active clinical trials. A Study to Test Asundexian for Preventing a Stroke Caused by a Clot in Participants After an Acute Ischemic Stroke or After a High-Risk Transient Ischemic Attack, a So-Called Mini Stroke (OCEANIC-STROKE) is examining the anticoagulant drug asundexian’s potential to prevent ischemic stroke in male and female adult patients, and A Phase 2 Trial of ALN-APP in Patients With Cerebral Amyloid Angiopathy (cAPPricorn-1) is evaluating the effect of ALN-APP on measures of CAA disease progression and characterizing the safety, tolerability, and pharmacodynamics of ALN-APP in adult patients with sporadic cerebral amyloid angiopathy (sCAA) and Dutch-type CAA (D-CAA).
